Advances in cell and gene therapies (CGT) offer the potential to transform medicine. They create an inflection point in the ability to treat and potentially cure many intractable illnesses. Since Novartis broke open the market in 2017 with Kymriah, followed a few months later by Gilead’s Yescarta, scientific development of CGT is booming and unlikely to slow down. The FDA is expecting to receive 200 applications in 2020 and predicts it will be approving 10 to 20 gene therapy products a year by 2025.
But there is a catch: each patient requires not only personalised treatment but a unique manufacturing process – and the industry is nowhere near ready. This is why we have invested in Ori Biotech.
The breakthrough success of CGT therapies is so recent that there is currently no way to reliably scale up production. The issue is only going to become more pressing; there are already more than 1,000 clinical trials, all competing for limited and inadequate production facilities. For perspective, the capacity of the largest facilities built by Gilead and Novartis struggle to produce a few hundred doses per year.
The few that do get manufactured then face the ultimate barrier. Due to current manufacturing set-ups, economies of scale don’t apply and existing treatments cost $500,000-$1,000,000 per patient. Payers are struggling to approve them.
Ori Biotech is building the solution. Let me explain why it’s needed.
What are Cell and Gene Therapies?
Cell therapy is the introduction of new cells into a patient’s body to grow, replace or repair damaged tissue in order to treat a disease. A variety of different types of cells can be used, including stem cells, lymphocytes, dendritic cells and pancreatic islet cells. Therapies can use cells from the patient’s own body (autologous) or from a donor (allogenic). Many therapies use adult cells that have been genetically reprogrammed and are capable of becoming one of many types of cells inside a patient’s body. This technology may enable the development of unlimited types of specific human cells needed for therapeutic purposes.
In some cases, such as chimeric antigen receptors (CAR-T), which essentially train immune system cells to kill off cancer cells, cells are genetically modified before being (re)introduced into the patient. This is the intersection between gene and cell therapy.
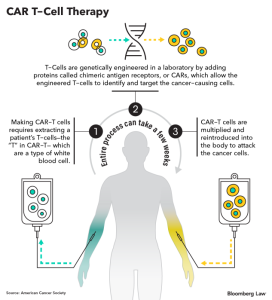
Gene therapy seeks to modify or introduce genes into a patient’s body with the goal of treating, preventing or potentially curing a disease. Examples of gene therapy approaches include replacing a mutated gene that causes disease with a functional copy or introducing a new, correct copy of a gene into the body. Therapeutic genes are packaged in a delivery vehicle, often deactivated viruses, and therapies may be performed in vivo, in which the therapeutic gene is directly delivered to cells inside the patient’s body, or ex vivo, in which the therapeutic gene is inserted into cells outside before being introduced into the body – these include several cell-based immunotherapies, such as CAR-T cell therapies, T-cell receptor (TCR) therapies, natural killer (NK) cell therapies, tumour-infiltrating lymphocytes (TILs), marrow-derived lymphocytes (MILs), gamma-delta T-cells and dendritic vaccines.
In recent years, cell and gene therapies, especially CAR-T, have been proven clinically to be life-saving therapies for several haematological cancers, and offer further promise for additional haematological conditions and solid tumours.
CGT Market Dynamics
The pharma industry is gearing up. Since 2015 there has been close to $40 billion of funding in the space and the market’s appetite for IPOs has dramatically increased, with companies raising an average of $200M (Autolus Therapeutics, AVROBIO, Magenta Therapeutics, Homology Medicines, Rubius Therapeutics and Orchard Therapeutics). What’s also unusual is how young they are. Allogene, for example, was created by a $300M Series A in April 2018, has a single candidate in phase 1 trials and was just 6 months old when its IPO raised a staggering $324M in October 2018 – the largest biotech IPO in almost a decade. At the end of 2019, Century Therapeutics launched with $250M to develop allogeneic cancer cell therapies.
FDA’s approval of Novartis’s CAR-T therapy, Kymriah, in August 2017 sparked a feeding frenzy. Gilead acquired another CAR-T developer, Kite Pharmaceuticals, for $12 billion, Celgene picked up Juno Therapeutics for $9 billion and Novartis bought AveXis for $8.7 billion. Celgene has since been acquired by Bristol-Myers Squibb for a massive $74 billion and the proposed takeovers of Luxturna’s manufacturer, Spark Therapeutics, by Roche for a reported $4.3 billion and Nightstar by Biogen for $800 million indicate the desire of large players to have a presence in the CGT market. You get the picture.
We anticipate digestion will prove difficult. The entire industry is facing a bottleneck – producing thousands of autologous products with individual processes is a new challenge facing pharmaceutical production. A large number of new therapies will aim to reach the market – only to be brought down by current production costs.
CGT Manufacturing Bottleneck
The single biggest hurdle to mass adoption of CGTs is their manufacturing and the logistics of getting the treatment to the patients, which all translate into untenable prices. This is exemplified by CAR-T products, which require the extraction, genetic modification, expansion and re-administration of each individual patient’s cells. This process involves transporting the cells from a hospital to a manufacturing facility and then back again, all under time pressure and with strict quality control.
In addition to the logistical challenges, cell therapy manufacturers must produce a consistently safe product from highly variable starting material. Patients who typically provide the raw material have different genetic backgrounds, stages of disease, and medical histories, all of which can introduce complexity into the production process.
In other words, the starting material is different every time, a situation quite different from the typical manufacturing scenario in which a well-defined cell line is used for each production run.
To scale up, CGT manufacturing processes must move from being open and manual to closed and automated, providing advantages including reduced contamination and risk, greater product consistency and efficiency, and more traceability throughout the process to ensure the chain of custody.
Most if not all cell therapies on the market are indicated for small patient populations, but if they are to become widely available across multiple indications, improvements in automation, process simplification, and supply chain management will be needed to meet demand. The goal of treating thousands of patients on an annual basis introduces entirely different challenges from the products and the manufacturing processes that have existed until now.
Payers Are Turning Up the Heat
Like any new treatment area, the long-term viability of CGT will ultimately hinge on affordability. Finding the balance between adequate returns and pricing that isn’t overly prohibitive is essential, particularly as more therapies are being developed for larger populations.
The current price tags are hard to stomach; namely $475,000 for Kymriah, $373,000 for Yescarta and $425,000 for Luxturna (per eye), posing a double challenge to payers, as they question whether they are paying the right price for the value.
It is estimated that around 60% of the costs stem from fixed production costs. The transformative potential of these therapeutics is motivating the industry to find solutions. As a result, performance-based pricing between manufacturers and payers, linking payment to the clinical and/or financial benefit seen in real-world conditions, are becoming prevalent.
They can operate at a patient level (the payer is refunded totally or partially if the patient does not meet response criteria) or at the population level (the payer is refunded partially, or the price decreases, if response across the population is less than would have been expected from trials). Due to the small patient numbers, patient-level approaches are more widespread. For example, Strimvelis is refunded if the patient (in Italy) is not “cured” and Kymriah (in the U.S.) is paid only if the patient responds after one month.
The reimbursement structure will depend on the type and size of the payer organisation, and whether the therapy is actually delivered over a short period of time. It has been proposed that distributing payments over time would allay the financial pressure. This formula could be implemented using various financial instruments, but adjustments in regulations and accounting rules may be needed in markets such as the U.S. or Germany where patients can move from one payer to another.
An interesting consideration is that annuitised payments can be combined with the ‘pay-by-results’ approach: installments are paid as long as the patient receives the benefits of the therapy (based on pre-specified criteria) or are adjusted over time.
Another model suggested for handling the financial burden and risks, whether CGTs or therapies for ultra-rare drugs in general, is the creation of a risk pool or dedicated fund. In the U.S., the idea has been floated of state-sponsored Medicaid pools or commercial elements, possibly coordinated and managed by an ‘Orphan Reinsurance and Benefit Manager’ (ORBM). In countries where a unique public payer is the norm, a ring-fenced fund might be considered. The U.K. recently agreed to fund Kymriah and Yescarta via the Cancer Drug Fund, but this pathway focuses on oncology therapies.
The take home message is straightforward: the economic burden is moving from payers to pharmas, who in turn are putting pressure on manufacturers to deliver scalable and cost-effective solutions.

This is where Ori Biotech comes in, the newest addition to the Amadeus early stage portfolio. The London- and Philadelphia-based cell and gene therapy manufacturing technology company rethinks how to manufacture this new class of therapeutics. The mission of the Ori platform is to fully automate cell and gene therapy manufacturing to increase throughput, improve quality and decrease costs in order to enable patient access to this new generation of life saving treatments.
Founded by Dr. Farlan Veraitch and Professor Chris Mason, the Company has brought together a seasoned Board and executive management team with over 80 years of pharmaceutical, cell therapy and venture building experience including CEO Jason C. Foster (Indivior) and CBO Jason Jones (Miltenyi Biotec) alongside industry-leading expert advisors like CAR-T poster-boy Bruce Levine and Anthony Davies.
Ori has developed a proprietary, flexible manufacturing platform that closes, automates and standardises manufacturing allowing therapeutics developers to further develop and bring their products from bench to bedside. The core technology will be used for all Cell and Gene therapy products ranging from today’s CAR-T products to gamma delta T cell and macrophage therapies of the future.

