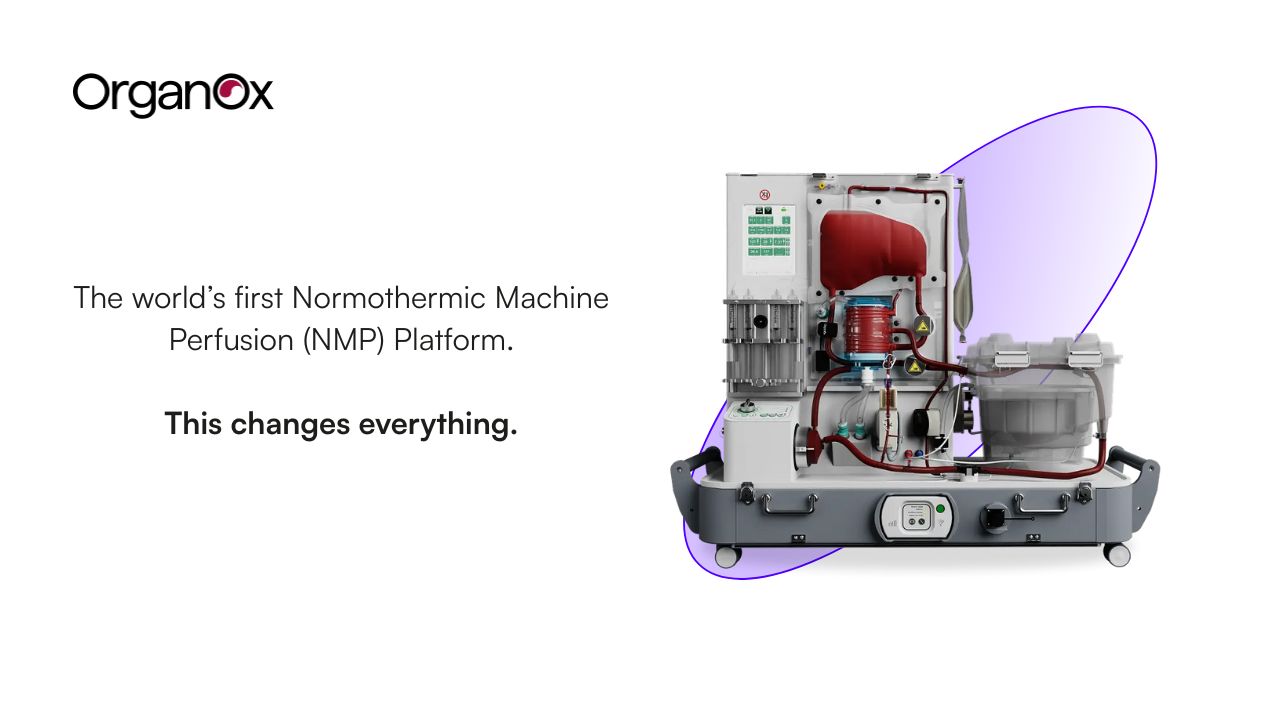
OrganOx turned a harsh reality into a system-level fix: too many donor livers never get transplanted. The company’s metra® platform keeps donor livers in a metabolically active state outside the body using normothermic machine perfusion (NMP) so that functional assessment of the organ can be performed prior to transplant, leading to an increased number of organs available for transplant and better allocation decisions. As of today, it has already supported 6,000+ liver transplants worldwide, with growing U.S. adoption and new operating approvals that expand logistics (including FDA approval for air transport).
Always looking ahead, the team is already working on NMP products to support additional organs, including kidneys, to extend this life saving technology to even more patient populations. With further development, NMP holds the potential to ultimately help heal organs inside or outside the body. Treating and potentially improving the quality of these organs may eventually enable clinicians to offer alternative therapies to patients for whom transplant is the only current life-saving option.
Why it matters
For patients, NMP has been shown to cut liver discards nearly in half (down to 12% of donated organs), while materially extending preservation time compared to static cold storage. This translates directly into more viable organs reaching more patients, and better long-term graft survival. For clinicians, it builds in flexibility: instead of middle-of-the-night emergency procedures, livers can be preserved for up to 24 hours, shifting surgeries to the daytime with full surgical teams. In practice, this means 84% of transplants now occur during the day with NMP vs. only 65% with cold storage.